Biomarker-Based Prediction of Tyrosine Kinase Inhibitor Cardiotoxicity using Human iPSC-Derived Cardiomyocytes [SPS 2019]
Presented by Jessica Palmer at the Safety Pharmacology Society Meeting in Barcelona, Spain (September, 2019)
Abstract
- Tyrosine kinase inhibitors (TKI) have greatly improved the treatment and prognosis for a wide range of cancers. Unfortunately, numerous TKIs produce cardiotoxic effects, which were not well predicted during preclinical studies.
- We developed an in vitro assay, Cardio quickPredict, for predicting cardiovascular liability based on changes in human iPSC-derived cardiomyocytes (iPSC-CM) metabolism and cell viability, which identifies both functional and structural cardiotoxicants. The assay’s prediction model (PM) is based on the response of four metabolites: lactic acid (LAC), arachidonic acid (ARA), thymidine (THY), and 2’-deoxycytidine (2DC)) and predicts the concentration at which a drug exhibits cardiotoxicity potential. The PM classified 81 drugs with known cardiotoxicity outcomes (54 cardiotoxic, 29 noncardiotoxic) with 86% balanced accuracy, 83% sensitivity, and 90% specificity.
- The current study evaluated the utility of this assay for evaluating the cardiotoxicity potential of TKIs. We tested 10 TKIs that induce a variety of cardiotoxic effects, including eight drugs clinically associated with cardiotoxicity (crizotinib, dasatinib, imatinib, lapatinib, nilotinib, sorafenib, sunitinib, and vandetanib) and two drugs considered to be relatively cardiac-safe (axitinib and erlotinib) to compare changes in metabolism of the PM ratios. Human iPSC-CMs were exposed to eight concentrations of each drug for 72 hours and cell viability and metabolites in the spent media were analyzed.
 Every drug altered at least one metabolite independent of a change in cell viability. Crizotinib, imatinib, sorafenib, sunitinib, and vandetanib elicited a response in all four metabolites indicative of cardiotoxicity; however, a difference was observed in which metabolite was impacted at the lowest concentration. For example, crizotinib altered LAC at significantly lower concentrations (?5-fold) than where a response was observed in ARA and THY. In contrast, sorafenib elicited a response in ARA prior to LAC, THY, and 2DC.
Every drug altered at least one metabolite independent of a change in cell viability. Crizotinib, imatinib, sorafenib, sunitinib, and vandetanib elicited a response in all four metabolites indicative of cardiotoxicity; however, a difference was observed in which metabolite was impacted at the lowest concentration. For example, crizotinib altered LAC at significantly lower concentrations (?5-fold) than where a response was observed in ARA and THY. In contrast, sorafenib elicited a response in ARA prior to LAC, THY, and 2DC.

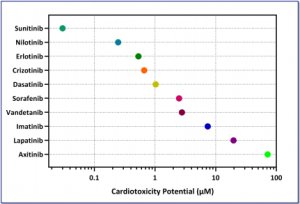 Every drug altered at least one metabolite independent of a change in cell viability. Crizotinib, imatinib, sorafenib, sunitinib, and vandetanib elicited a response in all four metabolites indicative of cardiotoxicity; however, a difference was observed in which metabolite was impacted at the lowest concentration. For example, crizotinib altered LAC at significantly lower concentrations (?5-fold) than where a response was observed in ARA and THY. In contrast, sorafenib elicited a response in ARA prior to LAC, THY, and 2DC.
Every drug altered at least one metabolite independent of a change in cell viability. Crizotinib, imatinib, sorafenib, sunitinib, and vandetanib elicited a response in all four metabolites indicative of cardiotoxicity; however, a difference was observed in which metabolite was impacted at the lowest concentration. For example, crizotinib altered LAC at significantly lower concentrations (?5-fold) than where a response was observed in ARA and THY. In contrast, sorafenib elicited a response in ARA prior to LAC, THY, and 2DC.
